3-Nitroaniline is an organic compound with the formula H2NC6H4NO2. A yellow solid, it is a derivative of aniline, carrying a nitro functional group in position 3. It is an isomer of 2-nitroaniline and 4-nitroaniline. It is used as a precursor to dyes.[1]
Synthesis and applications
3-Nitroaniline is produced on a commercial scale by reduction of 1,3-dinitrobenzene with hydrogen sulfide:[1]
In principle it can also be prepared by nitration of benzamide followed by the Hofmann rearrangement of the resulting 3-nitrobenzamide. The reaction involves treating the 3-nitrobenzamide with sodium hypobromite or sodium hypochlorite to transform the amide group into an amine.
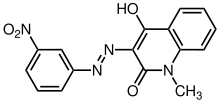
It is used as a chemical intermediate for azo coupling component 17 and the dyes disperse yellow 5 and acid blue 29. The chemical is changed to other substances (dyestuffs and m-nitrophenol) during the dyeing process.
References
- ^ a b Gerald Booth (2007). "Nitro Compounds, Aromatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_411. ISBN 978-3527306732.


Recent Comments