Isoprenol, also known as 3-methylbut-3-en-1-ol, is a hemiterpene alcohol. It is produced industrially as an intermediate to 3-methylbut-2-en-1-ol (prenol): global production in 2001 can be estimated as 6–13 thousand tons.[3]
Isoprenol is produced by the reaction between isobutene (2-methylpropene) and formaldehyde.

The thermodynamically preferred prenol with the more substituted double bond cannot be directly formed in the above reaction, but is produced via a subsequent isomerisation:
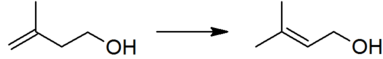
This isomerisation reaction is catalyzed by any species which can form an allyl complex without excessive hydrogenation of the substrate, for example poisoned palladium catalysts.[4]
Notes
- ^ Sigma-Aldrich Co. gives a value for the flash point of isoprenol of 42 °C (108 °F). The difference in the two values does not alter the safety classification of isoprenol as a category 3 flammable liquid under the GHS; but the lower value quoted here (from the New Zealand Environmental Risk Management Authority) would make it a class IC flammable liquid instead of a class II combustible liquid under the U.S. OSHA classification (29 C.F.R § 1910.106), and F3 rather than F2 under the NFPA 704 standard.
References
- ^ Sigma-Aldrich Co., 3-Methyl-3-buten-1-ol. Retrieved on 2009-08-31..
- ^ HSNO Chemical Classification Information Database, New Zealand Environmental Risk Management Authority, retrieved 2009-08-31.
- ^ 3-Methyl-2-buten-1-ol (PDF), SIDS Initial Assessment Report, Geneva: United Nations Environment Programme, May 2005. Major produce in a world is BASF(Germany) and Kuraray(Japan).
- ^ See, e.g., Kogan, S. B.; Kaliya, M.; Froumin, N. (2006), "Liquid phase isomerization of isoprenol into prenol in hydrogen environment", Appl. Catal. A: Gen., 297 (2): 231–36, doi:10.1016/j.apcata.2005.09.010.


Recent Comments