Eukaryote hybrid genomes result from interspecific hybridization, where closely related species mate and produce offspring with admixed genomes. The genomic revolution has shown that hybridization is common, and that it may represent an important source of novel variation. Although most interspecific hybrids are sterile or less fit than their parents, some may survive and reproduce, enabling the transfer of adaptive variants across the species boundary, and even result in the formation of novel evolutionary lineages. There are two main variants of hybrid species genomes: allopolyploid, which have one full chromosome set from each parent species, and homoploid, which are a mosaic of the parent species genomes with no increase in chromosome number. The establishment of hybrid species requires the development of reproductive isolation against parental species. Allopolyploid species often have strong intrinsic reproductive barriers due to differences in chromosome number, and homoploid hybrids can become reproductively isolated from the parent species through assortment of genetic incompatibilities. However, both types of hybrids can become further reproductively isolated, gaining extrinsic isolation barriers, by exploiting novel ecological niches, relative to their parents. Hybrids represent the merging of divergent genomes and thus face problems arising from incompatible combinations of genes. Thus hybrid genomes are highly dynamic and undergo rapid evolutionary change, including genome stabilization in which selection against incompatible combinations results in fixation of compatible ancestry block combinations within the hybrid species. The potential for rapid adapation or speciation makes hybrid genomes a particularly exciting subject of in evolutionary biology. Here we summarize how introgressed alleles or hybrid species can establish and how the resulting hybrid genomes evolve.
Background
Genetic exchange between species can impede the evolution of biodiversity because gene flow between diverging species counteracts their differentiation and hybridization between recently diverged species can lead to loss of genetic adaptations or species fusion.[1] Traditionally, zoologists have viewed interspecific hybridization as maladaptive behaviour[2] which can result in breaking up co-adapted gene complexes.[3] In contrast, plant biologists recognized early on that hybridization can sometimes be an important evolutionary force, contributing to increasing biodiversity.[4][5] Recently, evidence has been accumulating showing that hybridization is also an important evolutionary process in animals.[1][6][7] Interspecific hybridization can enrich the genetic diversity of introgressed taxon, lead to introgression of beneficial genetic variation or even generate new hybrid species.[1] Hybridization is now also known to contribute to the evolutionary potential in several textbook examples of adaptive radiation, including the Geospiza Galapagos finches,[8] African cichlid fishes,[9] Heliconius butterflies[10][11][12] and Hawaiian Madiinae tarweeds and silverswords.[13] Here we review the evolutionary outcomes of interspecific hybridization and the properties of genomes of hybrid genomes. Many of the discussed topics also apply to hybridization between different subspecies or populations of the same species, but here we focus on interspecific hybridization (referred to as hybridization in this review).
Evolutionary outcomes

There are several potential evolutionary outcomes of hybridization. If early generation hybrids are not viable or sterile, hybridization may reduce the reproductive success of the parent species.[14][15] This could potentially lead to reinforcement, selection to strengthen premating isolation[16] or if the species fail to evolve premating isolation, it could increase their extinction risk due to wasted reproductive effort.[14] If the fitness of early generation hybrids is non-zero and that of some later generation hybrids is as high or even higher than the fitness of one or both parent taxa, hybrids may displace the parent taxa and the hybridizing taxa may fuse (speciation reversal[17][18]). If the fitness of early generation hybrids is reduced but non-zero, hybrid zones may emerge in the contact zone of the taxa.[19] If hybrids are fertile, hybridization may contribute novel variation through rare hybrids backcrosssing with parental species. Such introgressive hybridization may enable neutral or selectively beneficial alleles to be transferred across species boundaries even in species pairs that remain distinct despite occasional gene flow.[20][21] Hybrid fitness may vary with divergence time between the hybridizing taxa. This pattern has been shown for a variety of taxa including Drosophila,[22] birds[23] and fish.[24] Hybrid fitness may also differ with cross direction,[25] between first generation and later generation hybrids,[26] and among individuals within generations of the same cross-type.[27][28] In some cases hybrids may evolve into new hybrid species with reproductive isolation to both parent taxa.[29][30] Below we describe the evolutionary outcomes of hybridisation that result in persistent hybrid genomes.
Adaptive introgression
When rare hybrids backcross with parent species alleles coding for traits that are beneficial for both parental species can be transferred across species boundaries, even if parent species remain distinct taxa. This process is referred to as adaptive introgression (a somewhat misleading term because backcrossing itself may not be adaptive, but some of the introgressed variants may be beneficial.[1]) Simulations suggest that adaptive introgression is possible unless hybrid fitness is substantially reduced,[31][32] or the adaptive loci are tightly linked to deleterious ones.[33] Examples of adaptive traits that have been transferred via introgression include an insecticide resistance gene that was transferred from Anopheles gambiae to A. coluzzii[21] and the red warning wing colouration trait in Heliconius butterflies that is under natural selection from predators which has been introgressed from e.g. H. melpomene to H. timareta[34] and other Heliconius species.[20] In the plant Arabidopsis arenosa some of the alleles conferring adaptation to drought and phytotoxic levels of metal have been introgressed from A. lyrata.[35] Even in humans there is evidence for adaptive introgression of e.g. immunity alleles, skin pigmentation alleles and alleles conferring adaptation to high altitude environments from Neanderthal and Denisovans.[36] If traits important for species recognition or reproductive isolation introgress into a population of another species, the introgressed population may become reproductively isolated against other populations of the same species. Examples of this include Heliconius butterflies, where selective introgression of wing pattern genes between diverged lineages occurs (see e.g.[37]), and wing patterns contribute to reproductive isolation in some species pairs with low (e.g. between H. t. florencia and H. t. linaresi) and intermediate levels (e.g. H. c. galanthus/H. pachinus) of divergence.[38]
Hybrid species definition
One of the potential evolutionary outcomes of hybridisation is the establishment of a novel, reproductively isolated lineage, i.e., hybrid speciation.[1][29] A hybrid species has an admixed genome and forms stable genetically distinct populations.[29] Some researchers argue that evidence of a hybridization-derived basis for reproductive isolation should be an additional defining criterion for hybrid speciation,[39] but see[40]. This stricter definition includes polyploid hybrid taxa but only encompasses a handful of well studied cases of homoploid hybrid speciation, e.g. Heliconius heurippa,[10][11][12] Passer italiae,[28] and three Helianthus sunflower species[41] because for most suggested examples of homoploid hybrid speciation, the genetic basis of reproductive isolation is still unknown.[39]
Hybrid species can occupy an ecological niche different to those of the parents and may be isolated from the parent species primarily through pre-mating barriers (hybrid speciation with external barriers, c.f. [42]). Hybrid species may also be reproductively isolated from the parent species through sorting of incompatibilities leading to new combinations of parental alleles that are incompatible with both parent species but compatible within the hybrid taxon (recombinational hybrid speciation).[29] A recombinational hybrid taxon typically also has a substantial proportion of the genome derived from the donor of introgressed material, although variation exists both between taxa and within lineages of hybrid taxa (see e.g.[43][44]).
Homoploid and polyploid hybrid speciation
In general, hybrid species can arise from two major types of hybrid speciation, defined by whether the speciation event is associated with genome duplication (polyploidy) or not. Homoploid hybrid speciation Homoploid hybrid speciation is defined as the evolution of a new hybrid species with reproductive isolation to both parent taxa without change of ploidy, i.e. number of chromosome sets (Fig. 2).[1] The genomes of homoploid hybrid species are mosaics of the parent genomes as ancestry tracts from the parent species are broken up by recombination.[40][41][45][46][47][48][49] In the case of polyploid hybrid speciation, hybridisation is associated with genome duplication, resulting in an allopolyploid with increased ploidy compared to their parental taxa (Fig. 2). In contrast to allopolyploids, autopolyploids are characterised by genome duplication within the same species and are thus not discussed further in the context of this review. Allopolyploid speciation is more common in plants than in animals.[50] Polyploid hybrids can be instantly isolated from their parental species through chromosome number differences.[50]
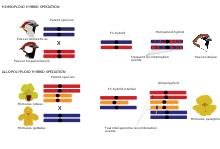
Reproductive isolation against parent species
Sufficient reproductive isolation from both parental species is required for the successful establishment of a hybrid species.[1][39][51] Reproductive isolation against parent species is harder to achieve for homoploid hybrids where karyotype differences do not contribute to intrinsic isolation. Reproductive isolation between a hybrid species and its parental species can arise from a variety of reproductive barriers either before or after fertilization (prezygotic or postzygotic, respectively), which may themselves be dependent or independent of environmental conditions (extrinsic or intrinsic barriers, respectively).[52] For example, intrinsic postzygotic barriers cause hybrid inviability or sterility regardless of the environment in which they occur, while extrinsic postzygotic barriers result in hybrids of low fitness due to maladaptation to specific environments.[30]
Prezygotic intrinsic and extrinsic differences have also been shown to be important in isolating hybrids from their parent species. In plants, pollinator mediated isolation resulting from changes in floral characteristics may be an important extrinsic prezygotic ecological barrier.[53][54][55][56] Strong extrinsic pre-zygotic has been shown to isolate the hybrid species Senecio eboracensis from its parent species, where hybrids are virtually absent in the wild, although a fraction of hybrid offspring are fertile in lab experiments.[57] Lowe & Abbott conclude that selfing, timing of flowering and characters involved in pollinator attraction likely contribute to this external isolation.[57] Prezygotic mate preference driven isolation generated from intrinsic assortative mating between hybrids has also been reported in several taxa. In African cichlid fish, experimental hybrids displayed combinations of parental traits and preferences which resulted in hybrids predominantly mating with other hybrids.[58] A similar pattern was found in Geospiza Galapagos finches where a specific hybrid song resulted from the transgressive beak morphology,[8] and hybrid Heliconius butterflies preferred the hybrid wing patterning over that of both parent species.[12] Intrinsic differences in habitat use[59] or in phenology[60] may result in some degree of reproductive isolation against parent species if mating is time and habitat-specific. For example the apple host race in Rhagoletis pomonella maggot flies evolved after introgression of diapause related genes from Mexican altiplano flies that allowed a switch from the ancestral host hawthorne to the later flowering apple [61][62] and isolated the two host races via allochronic intrinsic pre-zygotic isolation. In Xiphophorus swordtail fish strong ancestry assortative mating maintained a hybrid genetic cluster separate for 25 generations, but disappeared under manipulated conditions.[63] Hence, prezygotic reproductive barriers to gene flow may be environment dependent.
Postzygotic isolating barriers have also been shown to be important in a variety of hybrid lineages. Work on Helianthus sunflowers has revealed that intrinsic postzygotic can cause reproductive isolation against the parent species. The postzygotic barriers consist in pre-existing structural differences,[47][64] in combination with hybridization induced structural differences.[47] Sorting of incompatibilities between parent species, where one subset of these isolates the hybrid taxon against one parent and a different subset isolates it against the other parent, has resulted in intrinsic postzygotic isolation between the Italian sparrow Passer italiae and its parent species.[28] Simulation studies show that the likelihood of hybrid speciation through this mechanism depends on the divergence time between parent species,[65] the population size of the hybrid species,[66] the nature of selection acting on hybrids, and linkage among incompatibilities to each other and to adaptive variants.[67] Extrinsic ecological barriers against parent species may arise as by-products of ecological differentiation if mating is time and/or habitat specific. Hybrid species have been shown to adapt to novel ecological niches through transgressive phenotypes,[59] or through novel combinations of ecological traits from the parent species,[68] and ecological selection against parent-hybrid cross phenotypes would result in extrinsic postzygotic isolation.
Stabilization
Hybridization can have many different outcomes. Hybrid speciation results in reproductive isolation against both parent species and genomes that evolve independently from those of the parent species. Introgressive hybridization can transfer important novel variants into genomes of a species that remains distinct from the other taxa in spite of occasional gene flow. Here we refer to both types of hybridization-derived genomes as persistent hybrid genomes. Following initial hybridization, introgression tracts, the genetic blocks inherited from each parent species, are broken down with successive generations and recombination events. Recombination is more frequent in homoploid hybrid genomes than in allopolyploid hybrid genomes. In allopolyploids, recombination can destabilize the karyotype and lead to aberrant meiotic behaviour and reduced fertility, but may also generate novel gene combinations and advantageous phenotypic traits [69] as in homoploid hybrids. Once hybridization between the hybrid taxon and its parent taxa ceases, different ancestry blocks or introgression tracts may become fixed, a process referred to as "genome stabilization".[45] Some introgression tracts are removed by selection against incompatibilities and others are fixed. Theoretical models on hybrid zones suggest that the breakdown of ancestry blocks through recombination is suppressed near genes conferring reproductive isolation due to lower fitness of recombinant hybrids.[70] The strength of the suppression is affected by the form of selection, dominance, and whether the locus was situated on an autosome or sex chromosome.[70] The time to genome stabilization is variable. Fixation of ancestry blocks was found to be rapid in experimental hybrid Helianthus sunflower species genomes,[71] and the genome stabilization of hybrid sunflower species is estimated to take hundreds of generations.[45] In Zymoseptoria fungi genomes were stabilized within ca. 400 generations,[72] whereas in hybrid Xiphophorus swordtail genomes[73] genome stabilization was not achieved until after ca. 2000 and 2500 generations. Few Neanderthal regions have fixed in human genomes during the ca. 2000 generations after hybridization,[74] and segregating incompatibilities are present in the hybrid Italian sparrow approximately 5000 generations after the initial hybridization event.[75]
Given time, genetic drift will eventually stochastically fix blocks derived from the two parent species in finite isolated hybrid populations.[45] Selection against incompatibility loci may accelerate the process of fixation of parental alleles as hybrids that possess alleles that are less likely to cause incompatibility will have higher fitness and favourable alleles will spread in the population. Fixation of recessive weakly deleterious alleles in the parent taxa may, however, also result in hybrids retaining both parental alleles: because hybrids with haplotypes from both parents are not homozygous for any weakly deleterious alleles, they have higher fitness than hybrids with only one parental haplotype. This associative overdominance,[76][77] may slow down the process of fixation of parental alleles through favouring retention of both parental haplotypes. The effect of associative overdominance is strongest in low recombination regions, including inversions.[78] The balance between alleles and allelic combinations providing favourable phenotypic characters and the strength of selection against incompatibilities determine what introgression tracts will be inherited from which parent species upon hybridization (Fig.3).[21][79][80] An insecticide resistance region was retained following a hybridization event in Anopheles coluzzi,[21] suggesting a role for selection in maintaining favourable introgressed regions. The local recombination rate is important for the likelihood of introgression because in the case of widespread incompatibilities, introgressed alleles are more likely to recombine away from incompatibilities in high recombination regions. This pattern has been detected in monkeyflowers Mimulus,[81] in Mus domesticus house mice,[82] in Heliconius butterflies[80] and in Xiphophorus swordtail fish.[43]
Genome-wide incompatibilities have been identified in Xipophorous fish,[83] chimeric genes and mutations of orthologous genes cause incompatibilities in early generation experimental Cyprinidae goldfish - carp hybrids[84] and mito-nuclear incompatibilies are found to have a key role e.g. in Italian sparrows,[49][85] fungus[86] and cyto-nuclear incompatibilities in Mimulus plants.[87] Evidence from altered expression patterns in synthetic hybrids and missing gene combinations in a hybrid species also suggest that DNA-repair[49][84][88] and genes involved in mutagenesis and cancer related pathways[84] may cause incompatibilities in hybrids. Genome formation in hybrid species is shaped by selection against incompatible combinations.[43][73][79]
References
- ^ a b c d e f g Abbott, R.; Albach, D.; Ansell, S.; Arntzen, J. W.; Baird, S. J. E.; Bierne, N.; Boughman, J.; Brelsford, A.; Buerkle, C. A. (2013), "Hybridization and speciation", Journal of Evolutionary Biology, 26 (2): 229–246, doi:10.1111/j.1420-9101.2012.02599.x
- ^ Fisher, Ronald Aylmer (1930), The genetical theory of natural selection., Oxford: Clarendon Press, doi:10.5962/bhl.title.27468
{{citation}}: Text "book" ignored (help) - ^ Mayr, Ernst (1963), Animal Species and Evolution:, Cambridge, MA and London, England: Harvard University Press, doi:10.4159/harvard.9780674865327, ISBN 9780674865327
{{citation}}: Text "book" ignored (help) - ^ Stebbins, G. Ledyard (1959), "The Role of Hybridization in Evolution", Proceedings of the American Philosophical Society, 103 (2): 231–251, ISSN 0003-049X
- ^ Anderson, E.; Stebbins, G. L. (1954), "Hybridization as an evolutionary stimulus", Evolution, 8 (4): 378–388, doi:10.1111/j.1558-5646.1954.tb01504.x, ISSN 0014-3820
- ^ Arnold, Michael L. (1997), Natural Hybridization and Evolution., Cary: Oxford University Press, ISBN 9780195356687, OCLC 960164734
{{citation}}: Text "book" ignored (help) - ^ Mallet, James; Besansky, Nora; Hahn, Matthew W. (2016), "How reticulated are species?", BioEssays, 38 (2): 140–149, doi:10.1002/bies.201500149, PMC 4813508, PMID 26709836
{{citation}}: CS1 maint: PMC format (link) - ^ a b Lamichhaney, Sangeet; Han, Fan; Webster, Matthew T.; Andersson, Leif; Grant, B. Rosemary; Grant, Peter R. (2018), "Rapid hybrid speciation in Darwin's finches", Science, 359 (6372): 224–228, doi:10.1126/science.aao4593, ISSN 0036-8075
- ^ Meier, Joana I.; Marques, David A.; Mwaiko, Salome; Wagner, Catherine E.; Excoffier, Laurent; Seehausen, Ole (2017), "Ancient hybridization fuels rapid cichlid fish adaptive radiations", Nature Communications, 8 (1), doi:10.1038/ncomms14363, ISSN 2041-1723, PMC 5309898, PMID 28186104
{{citation}}: CS1 maint: PMC format (link) - ^ a b Mavárez, Jesús; Salazar, Camilo A.; Bermingham, Eldredge; Salcedo, Christian; Jiggins, Chris D.; Linares, Mauricio (2006), "Speciation by hybridization in Heliconius butterflies", Nature, 441 (7095): 868–871, doi:10.1038/nature04738, ISSN 0028-0836
- ^ a b Salazar, Camilo; Baxter, Simon W.; Pardo-Diaz, Carolina; Wu, Grace; Surridge, Alison; Linares, Mauricio; Bermingham, Eldredge; Jiggins, Chris D. (2010), Walsh, Bruce (ed.), "Genetic Evidence for Hybrid Trait Speciation in Heliconius Butterflies", PLoS Genetics, 6 (4): e1000930, doi:10.1371/journal.pgen.1000930, ISSN 1553-7404, PMC 2861694, PMID 20442862
{{citation}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ a b c Melo, Maria C.; Salazar, Camilo; Jiggins, Chris D.; Linares, Mauricio (2009), "Assortative mating preferences among hybrids offers a route to hybrid speciation", Evolution, 63 (6): 1660–1665, doi:10.1111/j.1558-5646.2009.00633.x
- ^ Tarweeds & silverswords : evolution of the Madiinae (Asteraceae), Carlquist, Sherwin John, 1930-, Baldwin, Bruce G., 1957-, Carr, Gerald D., St. Louis: Missouri Botanical Garden Press, 2003, ISBN 1930723202, OCLC 52892451
{{citation}}: Text "book" ignored (help)CS1 maint: others (link) - ^ a b Wolf, Diana E.; Takebayashi, Naoki; Rieseberg, Loren H. (2001), "Predicting the Risk of Extinction through Hybridization", Conservation Biology, 15 (4): 1039–1053, doi:10.1046/j.1523-1739.2001.0150041039.x, ISSN 0888-8892
- ^ Prentis, P. J.; White, E. M.; Radford, I. J.; Lowe, A. J.; Clarke, A. R. (2007), "Can hybridization cause local extinction: a case for demographic swamping of the Australian native Senecio pinnatifolius by the invasive Senecio madagascariensis?", New Phytologist, 176 (4): 902–912, doi:10.1111/j.1469-8137.2007.02217.x, ISSN 0028-646X
- ^ Servedio, Maria R.; Noor, Mohamed A.F. (2003), "The Role of Reinforcement in Speciation: Theory and Data", Annual Review of Ecology, Evolution, and Systematics, 34 (1): 339–364, doi:10.1146/annurev.ecolsys.34.011802.132412, ISSN 1543-592X
- ^ Rhymer, Judith M.; Simberloff, Daniel (1996), "EXTINCTION BY HYBRIDIZATION AND INTROGRESSION", Annual Review of Ecology and Systematics, 27 (1): 83–109, doi:10.1146/annurev.ecolsys.27.1.83, ISSN 0066-4162
- ^ Seehausen, Ole (2006), "Conservation: Losing Biodiversity by Reverse Speciation", Current Biology, 16 (9): R334–R337, doi:10.1016/j.cub.2006.03.080
- ^ Thompson, John D. (1994), "Harrison, R. G. (ed.). Hybrid Zones and the Evolutionary Process. Oxford University Press, Oxford. 364 pp. Price f45.00. ISBN: 0-19-506917-X.", Journal of Evolutionary Biology, 7 (5): 631–634, doi:10.1046/j.1420-9101.1994.7050631.x, ISSN 1010-061X
- ^ a b The Heliconius Genome Consortium (2012), "Butterfly genome reveals promiscuous exchange of mimicry adaptations among species", Nature, 487 (7405): 94–98, doi:10.1038/nature11041, ISSN 0028-0836, PMC 3398145, PMID 22722851
{{citation}}: CS1 maint: PMC format (link) - ^ a b c d Hanemaaijer, Mark J.; Collier, Travis C.; Chang, Allison; Shott, Chloe C.; Houston, Parker D.; Schmidt, Hanno; Main, Bradley J.; Cornel, Anthony J.; Lee, Yoosook (2018), "The fate of genes that cross species boundaries after a major hybridization event in a natural mosquito population", Molecular Ecology, 27 (24): 4978–4990, doi:10.1111/mec.14947
- ^ Coyne, Jerry A.; Orr, H. Allen (2004), Speciation, Sunderland: Sinauer Associates, ISBN 0878930914, OCLC 55078441
{{citation}}: Text "book" ignored (help) - ^ Price, Trevor D.; Bouvier, Michelle M. (2002), "The evolution of F1 postzygotic incompatibilities in birds", Evolution, 56 (10): 2083, doi:10.1554/0014-3820(2002)056[2083:teofpi]2.0.co;2, ISSN 0014-3820
- ^ Stelkens, Rike B.; Young, Kyle A.; Seehausen, Ole (2010), "The accumulation of reproductive incompatibilities in African cichlid fish", Evolution, 64 (3): 617–633, doi:10.1111/j.1558-5646.2009.00849.x
- ^ Rebernig, Carolin A.; Lafon-Placette, Clément; Hatorangan, Marcelinus R.; Slotte, Tanja; Köhler, Claudia (2015), Bomblies, Kirsten (ed.), "Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm", PLOS Genetics, 11 (6): e1005295, doi:10.1371/journal.pgen.1005295, ISSN 1553-7404, PMC 4472357, PMID 26086217
{{citation}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Pritchard, V. L.; Knutson, V. L.; Lee, M.; Zieba, J.; Edmands, S. (2013), "Fitness and morphological outcomes of many generations of hybridization in the copepod Tigriopus californicus", Journal of Evolutionary Biology, 26 (2): 416–433, doi:10.1111/jeb.12060
- ^ Rieseberg, Loren H; Archer, Margaret A; Wayne, Robert K (1999), "Transgressive segregation, adaptation and speciation", Heredity, 83 (4): 363–372, doi:10.1038/sj.hdy.6886170, ISSN 0018-067X
- ^ a b c Burke, John M.; Arnold, Michael L. (2001), "Genetics and the Fitness of Hybrids", Annual Review of Genetics, 35 (1): 31–52, doi:10.1146/annurev.genet.35.102401.085719, ISSN 0066-4197
- ^ a b c d Mallet, James (2007), "Hybrid speciation", Nature, 446 (7133): 279–283, doi:10.1038/nature05706, ISSN 0028-0836
- ^ a b Vallejo‐Marín, Mario; Hiscock, Simon J. (2016), "Hybridization and hybrid speciation under global change", New Phytologist, 211 (4): 1170–1187, doi:10.1111/nph.14004, ISSN 0028-646X
- ^ Barton, Nick; Bengtsson, Bengt Olle (1986), "The barrier to genetic exchange between hybridising populations", Heredity, 57 (3): 357–376, doi:10.1038/hdy.1986.135, ISSN 0018-067X
- ^ Demon, Inez; Haccou, Patsy; van den Bosch, Frank (2007), "Introgression of resistance genes between populations: A model study of insecticide resistance in Bemisia tabaci", Theoretical Population Biology, 72 (2): 292–304, doi:10.1016/j.tpb.2007.06.005
- ^ Uecker, Hildegard; Setter, Derek; Hermisson, Joachim (2015), "Adaptive gene introgression after secondary contact", Journal of Mathematical Biology, 70 (7): 1523–1580, doi:10.1007/s00285-014-0802-y, ISSN 0303-6812, PMC 4426140, PMID 24992884
{{citation}}: CS1 maint: PMC format (link) - ^ Pardo-Diaz, Carolina; Salazar, Camilo; Baxter, Simon W.; Merot, Claire; Figueiredo-Ready, Wilsea; Joron, Mathieu; McMillan, W. Owen; Jiggins, Chris D. (2012), R. Kronforst, Marcus (ed.), "Adaptive Introgression across Species Boundaries in Heliconius Butterflies", PLoS Genetics, 8 (6): e1002752, doi:10.1371/journal.pgen.1002752, ISSN 1553-7404, PMC 3380824, PMID 22737081
{{citation}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Arnold, Brian J.; Lahner, Brett; DaCosta, Jeffrey M.; Weisman, Caroline M.; Hollister, Jesse D.; Salt, David E.; Bomblies, Kirsten; Yant, Levi (2016), "Borrowed alleles and convergence in serpentine adaptation", Proceedings of the National Academy of Sciences, 113 (29): 8320–8325, doi:10.1073/pnas.1600405113, ISSN 0027-8424, PMC 4961121, PMID 27357660
{{citation}}: CS1 maint: PMC format (link) - ^ Racimo, Fernando; Sankararaman, Sriram; Nielsen, Rasmus; Huerta-Sánchez, Emilia (2015), "Evidence for archaic adaptive introgression in humans", Nature Reviews Genetics, 16 (6): 359–371, doi:10.1038/nrg3936, ISSN 1471-0056, PMC 4478293, PMID 25963373
{{citation}}: CS1 maint: PMC format (link) - ^ Kronforst, M. R.; Papa, R. (2015), "The Functional Basis of Wing Patterning in Heliconius Butterflies: The Molecules Behind Mimicry", Genetics, 200 (1): 1–19, doi:10.1534/genetics.114.172387, ISSN 0016-6731, PMC 4423356, PMID 25953905
{{citation}}: CS1 maint: PMC format (link) - ^ Mérot, C.; Salazar, C.; Merrill, R. M.; Jiggins, C. D.; Joron, M. (2017), "What shapes the continuum of reproductive isolation? Lessons from Heliconius butterflies", Proceedings of the Royal Society B: Biological Sciences, 284 (1856): 20170335, doi:10.1098/rspb.2017.0335, ISSN 0962-8452, PMC 5474069, PMID 28592669
{{citation}}: CS1 maint: PMC format (link) - ^ a b c Schumer, Molly; Rosenthal, Gil G.; Andolfatto, Peter (2014), "How common is homoploid hybrid speciation", Evolution, 68 (6): 1553–1560, doi:10.1111/evo.12399
- ^ a b Nieto Feliner, G; Álvarez, I; Fuertes-Aguilar, J; Heuertz, M; Marques, I; Moharrek, F; Piñeiro, R; Riina, R; Rosselló, J A (2017), "Is homoploid hybrid speciation that rare? An empiricist's view", Heredity, 118 (6): 513–516, doi:10.1038/hdy.2017.7, ISSN 0018-067X, PMC 5436029, PMID 28295029
{{citation}}: CS1 maint: PMC format (link) - ^ a b Rieseberg, L. H. (2003), "Major Ecological Transitions in Wild Sunflowers Facilitated by Hybridization", Science, 301 (5637): 1211–1216, doi:10.1126/science.1086949, ISSN 0036-8075
- ^ Grant, Verne. (1981), Plant speciation (2nd ed ed.), New York: Columbia University Press, ISBN 0231051123, OCLC 7552165
{{citation}}:|edition=has extra text (help); Text "book" ignored (help) - ^ a b c Schumer, Molly; Xu, Chenling; Powell, Daniel L.; Durvasula, Arun; Skov, Laurits; Holland, Chris; Blazier, John C.; Sankararaman, Sriram; Andolfatto, Peter (2018), "Natural selection interacts with recombination to shape the evolution of hybrid genomes", Science, 360 (6389): 656–660, doi:10.1126/science.aar3684, ISSN 0036-8075, PMC 6069607, PMID 29674434
{{citation}}: CS1 maint: PMC format (link) - ^ Runemark, Anna; Trier, Cassandra N.; Eroukhmanoff, Fabrice; Hermansen, Jo S.; Matschiner, Michael; Ravinet, Mark; Elgvin, Tore O.; Sætre, Glenn-Peter (2018), "Variation and constraints in hybrid genome formation", Nature Ecology & Evolution, 2 (3): 549–556, doi:10.1038/s41559-017-0437-7, ISSN 2397-334X
- ^ a b c d Buerkle, C. Alex; Rieseberg, Loren H. (2008), "The rate of genome stabilization in homoploid hybrid species", Evolution, 62 (2): 266–275, doi:10.1111/j.1558-5646.2007.00267.x, ISSN 0014-3820, PMC 2442919, PMID 18039323
{{citation}}: CS1 maint: PMC format (link) - ^ Ungerer, M. C.; Baird, S. J. E.; Pan, J.; Rieseberg, L. H. (1998), "Rapid hybrid speciation in wild sunflowers", Proceedings of the National Academy of Sciences, 95 (20): 11757–11762, doi:10.1073/pnas.95.20.11757, ISSN 0027-8424, PMC 21713, PMID 9751738
{{citation}}: CS1 maint: PMC format (link) - ^ a b c Lai, Zhao; Nakazato, Takuya; Salmaso, Marzia; Burke, John M.; Tang, Shunxue; Knapp, Steven J.; Rieseberg, Loren H. (2005), "Extensive Chromosomal Repatterning and the Evolution of Sterility Barriers in Hybrid Sunflower Species", Genetics, 171 (1): 291–303, doi:10.1534/genetics.105.042242, ISSN 0016-6731, PMC 1456521, PMID 16183908
{{citation}}: CS1 maint: PMC format (link) - ^ Elgvin, Tore O.; Trier, Cassandra N.; Tørresen, Ole K.; Hagen, Ingerid J.; Lien, Sigbjørn; Nederbragt, Alexander J.; Ravinet, Mark; Jensen, Henrik; Sætre, Glenn-Peter (2017), "The genomic mosaicism of hybrid speciation", Science Advances, 3 (6): e1602996, doi:10.1126/sciadv.1602996, ISSN 2375-2548, PMC 5470830, PMID 28630911
{{citation}}: CS1 maint: PMC format (link) - ^ a b c Runemark, Anna; Trier, Cassandra N.; Eroukhmanoff, Fabrice; Hermansen, Jo S.; Matschiner, Michael; Ravinet, Mark; Elgvin, Tore O.; Sætre, Glenn-Peter (2018), "Variation and constraints in hybrid genome formation", Nature Ecology & Evolution, 2 (3): 549–556, doi:10.1038/s41559-017-0437-7, ISSN 2397-334X
- ^ a b Otto, Sarah P; Whitton, Jeannette (2000), "Polyploid Incidence and Evolution", Annual Review of Genetics, 34 (1): 401–437, doi:10.1146/annurev.genet.34.1.401, ISSN 0066-4197
- ^ Abbott, Richard J; Rieseberg, Loren H (2012), John Wiley & Sons, Ltd (ed.), "Hybrid Speciation", eLS, John Wiley & Sons, Ltd, doi:10.1002/9780470015902.a0001753.pub2, ISBN 9780470016176
- ^ Coyne, Jerry A (1989), "Mutation rates in hybrids between sibling species of Drosophila", Heredity, 63 (2): 155–162, doi:10.1038/hdy.1989.87, ISSN 0018-067X
- ^ Chase, Mark W; Paun, Ovidiu; Fay, Michael F (2010), "Hybridization and speciation in angiosperms: arole for pollinator shifts?", Journal of Biology, 9 (3): 21, doi:10.1186/jbiol231, ISSN 1475-4924
{{citation}}: CS1 maint: unflagged free DOI (link) - ^ Grant, Verne (1949), "Pollination systems as isolating mechanisms in angiosperms", Evolution, 3 (1): 82–97, doi:10.1111/j.1558-5646.1949.tb00007.x
- ^ Segraves, K. A.; Thompson, J. N. (1999), "Plant polyploidy and pollination: floral traits and insect visits to diploid and tetraploid Heuchera grossulariifolia", Evolution, 53 (4): 1114–1127, doi:10.1111/j.1558-5646.1999.tb04526.x
- ^ Moe, Annika M.; Weiblen, George D. (2012), "Pollinator-mediated reproductive isolation among dioecious fig species (Ficus, Moraceae)", Evolution, 66 (12): 3710–3721, doi:10.1111/j.1558-5646.2012.01727.x
- ^ a b Lowe, A J; Abbott, R J (2004), "Reproductive isolation of a new hybrid species, Senecio eboracensis Abbott & Lowe (Asteraceae)", Heredity, 92 (5): 386–395, doi:10.1038/sj.hdy.6800432, ISSN 0018-067X
- ^ Selz, O. M.; Thommen, R.; Maan, M. E.; Seehausen, O. (2014), "Behavioural isolation may facilitate homoploid hybrid speciation in cichlid fish", Journal of Evolutionary Biology, 27 (2): 275–289, doi:10.1111/jeb.12287
- ^ a b Schwarzbach, Andrea E.; Donovan, Lisa A.; Rieseberg, Loren H. (2001), "Transgressive character expression in a hybrid sunflower species", American Journal of Botany, 88 (2): 270–277, doi:10.2307/2657018, ISSN 0002-9122
- ^ Mameli, Giulia; López-Alvarado, Javier; Farris, Emmanuele; Susanna, Alfonso; Filigheddu, Rossella; Garcia-Jacas, Núria (2014), "The role of parental and hybrid species in multiple introgression events: evidence of homoploid hybrid speciation in Centaurea (Cardueae, Asteraceae): Introgression in Centaurea", Botanical Journal of the Linnean Society, 175 (3): 453–467, doi:10.1111/boj.12177
- ^ Xie, X.; Michel, A. P.; Schwarz, D.; Rull, J.; Velez, S.; Forbes, A. A.; Aluja, M.; Feder, J. L. (2008), "Radiation and divergence in the Rhagoletis Pomonella species complex: inferences from DNA sequence data", Journal of Evolutionary Biology, 21 (3): 900–913, doi:10.1111/j.1420-9101.2008.01507.x, ISSN 1010-061X
- ^ Feder, J. L.; Xie, X.; Rull, J.; Velez, S.; Forbes, A.; Leung, B.; Dambroski, H.; Filchak, K. E.; Aluja, M. (2005), "Mayr, Dobzhansky, and Bush and the complexities of sympatric speciation in Rhagoletis", Proceedings of the National Academy of Sciences, 102 (Supplement 1): 6573–6580, doi:10.1073/pnas.0502099102, ISSN 0027-8424, PMC 1131876, PMID 15851672
{{citation}}: CS1 maint: PMC format (link) - ^ Schumer, Molly; Powell, Daniel L.; Delclós, Pablo J.; Squire, Mattie; Cui, Rongfeng; Andolfatto, Peter; Rosenthal, Gil G. (2017), "Assortative mating and persistent reproductive isolation in hybrids", Proceedings of the National Academy of Sciences, 114 (41): 10936–10941, doi:10.1073/pnas.1711238114, ISSN 0027-8424, PMC 5642718, PMID 28973863
{{citation}}: CS1 maint: PMC format (link) - ^ Rieseberg, L. H.; Linder, C. R.; Seiler, G. J. (1995), "Chromosomal and genic barriers to introgression in Helianthus", Genetics, 141 (3): 1163–1171, ISSN 0016-6731, PMC 1206838, PMID 8582621
- ^ Comeault, Aaron A.; Matute, Daniel R. (2018), "Genetic divergence and the number of hybridizing species affect the path to homoploid hybrid speciation", Proceedings of the National Academy of Sciences, 115 (39): 9761–9766, doi:10.1073/pnas.1809685115, ISSN 0027-8424, PMC 6166845, PMID 30209213
{{citation}}: CS1 maint: PMC format (link) - ^ Blanckaert, Alexandre; Bank, Claudia (2018), Zhang, Jianzhi (ed.), "In search of the Goldilocks zone for hybrid speciation", PLOS Genetics, 14 (9): e1007613, doi:10.1371/journal.pgen.1007613, ISSN 1553-7404, PMC 6145587, PMID 30192761
{{citation}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Schumer, Molly; Cui, Rongfeng; Rosenthal, Gil G.; Andolfatto, Peter (2015), Payseur, Bret A. (ed.), "Reproductive Isolation of Hybrid Populations Driven by Genetic Incompatibilities", PLOS Genetics, 11 (3): e1005041, doi:10.1371/journal.pgen.1005041, ISSN 1553-7404, PMC 4359097, PMID 25768654
{{citation}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Vereecken, Nicolas J; Cozzolino, Salvatore; Schiestl, Florian P (2010), "Hybrid floral scent novelty drives pollinator shift in sexually deceptive orchids", BMC Evolutionary Biology, 10 (1): 103, doi:10.1186/1471-2148-10-103, ISSN 1471-2148, PMC 2875231, PMID 20409296
{{citation}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Gaeta, Robert T.; Chris Pires, J. (2010), "Homoeologous recombination in allopolyploids: the polyploid ratchet: Research review", New Phytologist, 186 (1): 18–28, doi:10.1111/j.1469-8137.2009.03089.x
- ^ a b Hvala, John A.; Frayer, Megan E.; Payseur, Bret A. (2018), "Signatures of hybridization and speciation in genomic patterns of ancestry", Evolution, 72 (8): 1540–1552, doi:10.1111/evo.13509, PMC 6261709, PMID 29806154
{{citation}}: CS1 maint: PMC format (link) - ^ Rieseberg, L. H.; Sinervo, B.; Linder, C. R.; Ungerer, M. C.; Arias, D. M. (1996), "Role of Gene Interactions in Hybrid Speciation: Evidence from Ancient and Experimental Hybrids", Science, 272 (5262): 741–745, doi:10.1126/science.272.5262.741, ISSN 0036-8075
- ^ Stukenbrock, E. H.; Christiansen, F. B.; Hansen, T. T.; Dutheil, J. Y.; Schierup, M. H. (2012), "Fusion of two divergent fungal individuals led to the recent emergence of a unique widespread pathogen species", Proceedings of the National Academy of Sciences, 109 (27): 10954–10959, doi:10.1073/pnas.1201403109, ISSN 0027-8424, PMC 3390827, PMID 22711811
{{citation}}: CS1 maint: PMC format (link) - ^ a b Schumer, Molly; Brandvain, Yaniv (2016), "Determining epistatic selection in admixed populations", Molecular Ecology, 25 (11): 2577–2591, doi:10.1111/mec.13641
- ^ Sankararaman, Sriram; Mallick, Swapan; Dannemann, Michael; Prüfer, Kay; Kelso, Janet; Pääbo, Svante; Patterson, Nick; Reich, David (2014), "The genomic landscape of Neanderthal ancestry in present-day humans", Nature, 507 (7492): 354–357, doi:10.1038/nature12961, ISSN 0028-0836, PMC 4072735, PMID 24476815
{{citation}}: CS1 maint: PMC format (link) - ^ Eroukhmanoff, Fabrice; Bailey, Richard I.; Elgvin, Tore O.; Hermansen, Jo S.; Runemark, Anna R.; Trier, Cassandra N.; Sætre, Glenn-Peter (2017), "Resolution of conflict between parental genomes in a hybrid species", bioRxiv, doi:10.1101/102970
- ^ Ohta, Tomoko (1971), "Associative overdominance caused by linked detrimental mutations", Genetical Research, 18 (3): 277–286, doi:10.1017/s0016672300012684, ISSN 0016-6723
- ^ Zhao, Lei; Charlesworth, Brian (2016), "Resolving the Conflict Between Associative Overdominance and Background Selection", Genetics, 203 (3): 1315–1334, doi:10.1534/genetics.116.188912, ISSN 0016-6731, PMC 4937488, PMID 27182952
{{citation}}: CS1 maint: PMC format (link) - ^ Faria, Rui; Johannesson, Kerstin; Butlin, Roger K.; Westram, Anja M. (2019), "Evolving Inversions", Trends in Ecology & Evolution, 34 (3): 239–248, doi:10.1016/j.tree.2018.12.005
- ^ a b Barton, Nicholas H. (2018), "The consequences of an introgression event", Molecular Ecology, 27 (24): 4973–4975, doi:10.1111/mec.14950
- ^ a b Martin, Simon H.; Davey, John W.; Salazar, Camilo; Jiggins, Chris D. (2019), Moyle, Leonie (ed.), "Recombination rate variation shapes barriers to introgression across butterfly genomes", PLOS Biology, 17 (2): e2006288, doi:10.1371/journal.pbio.2006288, ISSN 1545-7885, PMC 6366726, PMID 30730876
{{citation}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Brandvain, Yaniv; Kenney, Amanda M.; Flagel, Lex; Coop, Graham; Sweigart, Andrea L. (2014), Jiggins, Chris D. (ed.), "Speciation and Introgression between Mimulus nasutus and Mimulus guttatus", PLoS Genetics, 10 (6): e1004410, doi:10.1371/journal.pgen.1004410, ISSN 1553-7404, PMC 4072524, PMID 24967630
{{citation}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Janoušek, Václav; Munclinger, Pavel; Wang, Liuyang; Teeter, Katherine C.; Tucker, Priscilla K. (2015), "Functional Organization of the Genome May Shape the Species Boundary in the House Mouse", Molecular Biology and Evolution, 32 (5): 1208–1220, doi:10.1093/molbev/msv011, ISSN 1537-1719, PMC 4408407, PMID 25631927
{{citation}}: CS1 maint: PMC format (link) - ^ Schumer, Molly; Cui, Rongfeng; Powell, Daniel L; Dresner, Rebecca; Rosenthal, Gil G; Andolfatto, Peter (2014), "High-resolution mapping reveals hundreds of genetic incompatibilities in hybridizing fish species", eLife, 3, doi:10.7554/eLife.02535, ISSN 2050-084X, PMC 4080447, PMID 24898754
{{citation}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ a b c Liu, Shaojun; Luo, Jing; Chai, Jing; Ren, Li; Zhou, Yi; Huang, Feng; Liu, Xiaochuan; Chen, Yubao; Zhang, Chun (2016), "Genomic incompatibilities in the diploid and tetraploid offspring of the goldfish × common carp cross", Proceedings of the National Academy of Sciences, 113 (5): 1327–1332, doi:10.1073/pnas.1512955113, ISSN 0027-8424, PMC 4747765, PMID 26768847
{{citation}}: CS1 maint: PMC format (link) - ^ Trier, Cassandra N.; Hermansen, Jo S.; Sætre, Glenn-Peter; Bailey, Richard I. (2014), Jiggins, Chris D. (ed.), "Evidence for Mito-Nuclear and Sex-Linked Reproductive Barriers between the Hybrid Italian Sparrow and Its Parent Species", PLoS Genetics, 10 (1): e1004075, doi:10.1371/journal.pgen.1004075, ISSN 1553-7404, PMC 3886922, PMID 24415954
{{citation}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Giordano, Luana; Sillo, Fabiano; Garbelotto, Matteo; Gonthier, Paolo (2018), "Mitonuclear interactions may contribute to fitness of fungal hybrids", Scientific Reports, 8 (1), doi:10.1038/s41598-018-19922-w, ISSN 2045-2322, PMC 5786003, PMID 29374209
{{citation}}: CS1 maint: PMC format (link) - ^ Case, Andrea L.; Finseth, Findley R.; Barr, Camille M.; Fishman, Lila (2016), "Selfish evolution of cytonuclear hybrid incompatibility in Mimulus", Proceedings of the Royal Society B: Biological Sciences, 283 (1838): 20161493, doi:10.1098/rspb.2016.1493, ISSN 0962-8452, PMC 5031664, PMID 27629037
{{citation}}: CS1 maint: PMC format (link) - ^ David, Wendi M.; Mitchell, David L.; Walter, Ronald B. (2004), "DNA repair in hybrid fish of the genus Xiphophorus", Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 138 (3): 301–309, doi:10.1016/j.cca.2004.07.006


Recent Comments