How Can We Help?
Gold(III) fluoride, AuF3, is an orange solid that sublimes at 300 °C.[4] It is a powerful fluorinating agent. It is very sensitive to moisture, yielding gold(III) hydroxide and hydrofluoric acid.
Preparation
AuF3 can be prepared by reacting AuCl3 with F2 or BrF3.[3]
Structure
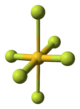
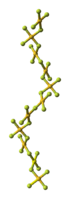
The crystal structure of AuF3 consists of spirals of square-planar AuF4 units.[5]
 |
 |
 |
 |
|
| AuF3 unit cell | neighbouring (AuF3)n helices | distorted octahedral coordination of gold by six fluorines | top-down view of an (AuF3)n helix | side view of an (AuF3)n helix |
References
- ^ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. pp. 4–59. ISBN 0-8493-0594-2.
- ^ Victor Lenher (1903). "Fluoride of Gold.1". Journal of the American Chemical Society. 25 (11): 1136–1138. doi:10.1021/ja02013a004.
- ^ a b Inis C. Tornieporth-Oetting; Thomas M. Klapötke (1995). "Laboratory Scale Direct Synthesis of Pure AuF3". Chemische Berichte. 128 (9): 957–958. doi:10.1002/cber.19951280918.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8., p. 1184.
- ^ F. W. B. Einstein; P. R. Rao; James Trotter; Neil Bartlett (1967). "The crystal structure of gold trifluoride". Journal of the Chemical Society A: Inorganic, Physical, Theoretical. 4: 478–482. doi:10.1039/J19670000478.
External links
 Media related to Gold trifluoride at Wikimedia Commons
Media related to Gold trifluoride at Wikimedia Commons


Recent Comments