Mesityl oxide is a α,β-unsaturated ketone with the formula CH3C(O)CH=C(CH3)2. This compound is a colorless, volatile liquid with a honey-like odor.[3]
Synthesis
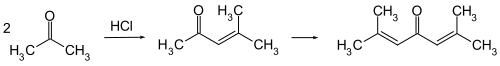
It is prepared by the aldol condensation of acetone to give diacetone alcohol, which readily dehydrates to give this compound.[4][5]
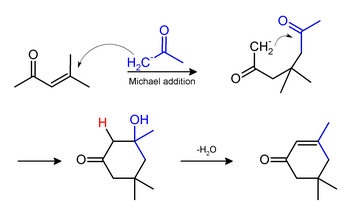
Phorone and isophorone may be formed under the same conditions. Isophorone originates via a Michael addition:
Phorone is formed by continued aldol condensation:
Uses
Mesityl oxide is used as a solvent and in the production of methyl isobutyl ketone by hydrogenation:[5]
Further hydrogenation gives 4-methyl-2-pentanol (methyl isobutyl carbinol).
Dimedone is another established use of mesityl oxide.
References
- ^ a b c d e f g h i NIOSH Pocket Guide to Chemical Hazards. "#0385". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "Mesityl oxide". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Merck Index, 14th Edition
- ^ Conant, J. B.; Tuttle, N. (1921). "Mesityl Oxide". Organic Syntheses. 1: 53. doi:10.15227/orgsyn.001.0053.
- ^ a b Sifniades, Stylianos; Levy, Alan B. (2000). "Acetone". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a01_079. ISBN 3527306730.




Recent Comments